Periodic table quiz 1 36 – Embark on an exciting journey with our periodic table quiz 1-36! This interactive adventure will guide you through the fascinating world of the first 36 elements, revealing their properties, behaviors, and real-world applications.
Prepare to delve into the periodic table’s intricate structure, unravel the secrets of element classification, witness the dynamics of chemical reactions, and uncover the practical uses of these essential building blocks of our universe.
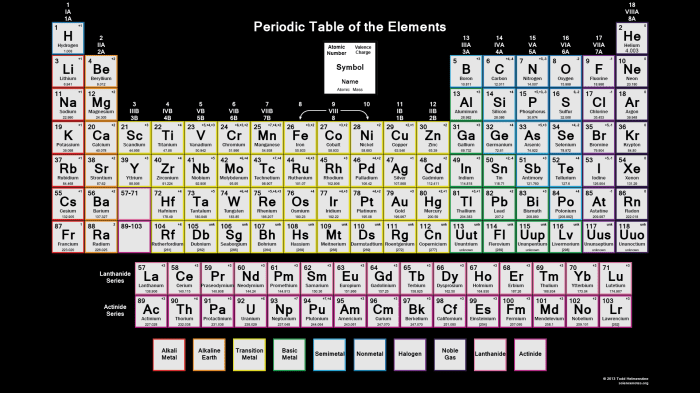
Periodic Table Structure
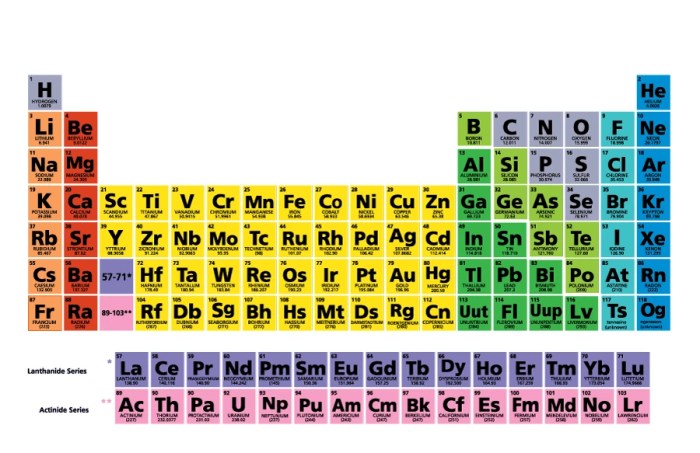
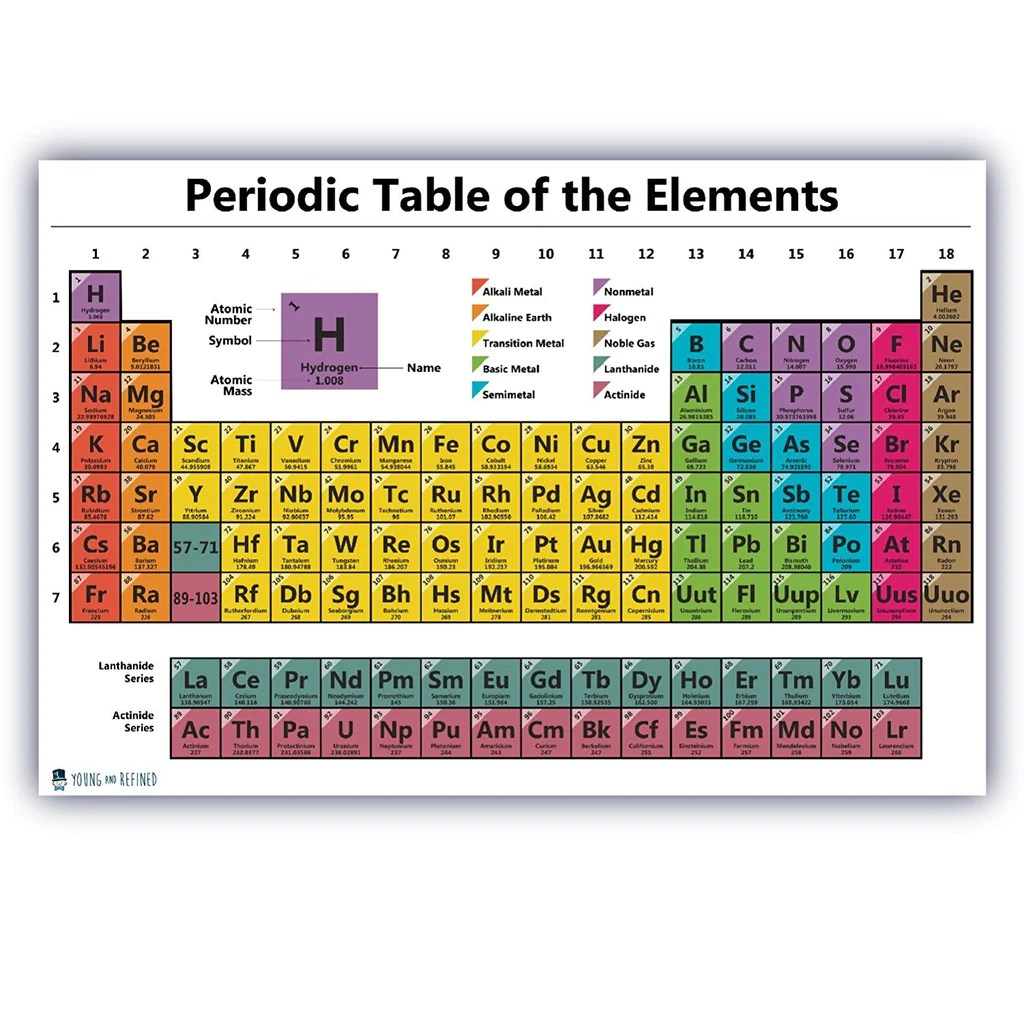
The periodic table is a tabular arrangement of chemical elements, organized on the basis of their atomic number, electron configuration, and recurring chemical properties. It is generally accepted that the modern periodic table was first published by Dmitri Mendeleev in 1869, although several other scientists had developed similar tables prior to this.The
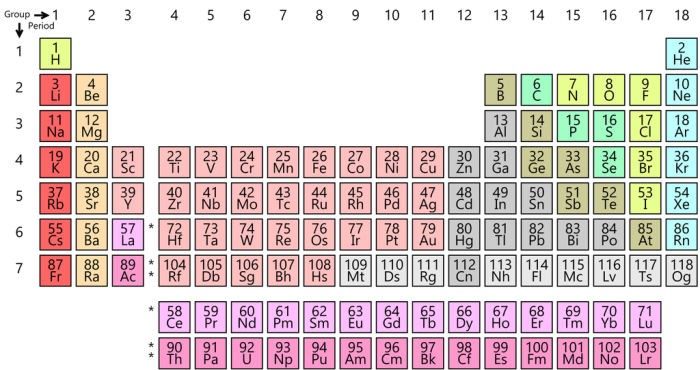
periodic table is divided into 18 vertical columns, called groups, and 7 horizontal rows, called periods. The groups are numbered 1-18 from left to right, and the periods are numbered 1-7 from top to bottom.Elements in the same group have similar chemical properties because they have the same number of valence electrons.
Valence electrons are the electrons in the outermost shell of an atom, and they determine the chemical reactivity of the element.Elements in the same period have the same number of electron shells. As you move from left to right across a period, the atomic number increases, and the elements become more electronegative.
Electronegativity is a measure of an atom’s ability to attract electrons.The periodic table can be used to predict the properties of an element based on its position in the table. For example, an element in the same group as sodium will likely be a soft, silvery metal that reacts easily with water.
An element in the same period as oxygen will likely be a gas at room temperature.
Element Properties and Classification
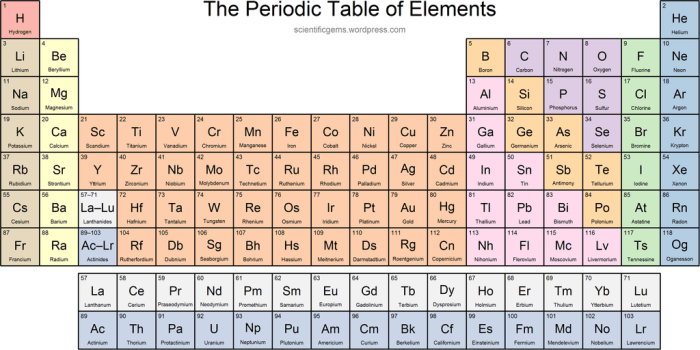
The first 36 elements in the periodic table exhibit a diverse range of properties and can be classified into distinct groups based on their chemical behavior. Understanding these properties and classifications provides a solid foundation for exploring the chemistry of elements and their interactions.
Key Properties of Elements in the First 36 Positions
- Atomic Number:The number of protons in an element’s nucleus, which determines its position in the periodic table.
- Atomic Mass:The average mass of an element’s atoms, including both protons and neutrons.
- Electron Configuration:The arrangement of electrons in an element’s orbitals, which influences its chemical properties.
- Electronegativity:A measure of an element’s ability to attract electrons in a chemical bond.
- Ionization Energy:The energy required to remove an electron from an atom.
- Metallic Character:The tendency of an element to lose electrons and form positive ions.
Classification of Elements
Based on their chemical behavior, the first 36 elements can be classified into several groups:
- Alkali Metals:(Group 1) Highly reactive metals that readily form 1+ ions. (e.g., Lithium, Sodium)
- Alkaline Earth Metals:(Group 2) Less reactive metals that form 2+ ions. (e.g., Magnesium, Calcium)
- Halogens:(Group 17) Highly reactive non-metals that form 1- ions. (e.g., Fluorine, Chlorine)
- Noble Gases:(Group 18) Inert gases that do not form chemical bonds. (e.g., Helium, Neon)
Relationship between Position and Properties
The position of an element in the periodic table provides valuable insights into its properties. Elements in the same group share similar chemical properties due to having the same number of valence electrons. As you move across a period (row) from left to right, the elements become more electronegative and metallic character decreases.
Reactivity and Chemical Reactions
Reactivity is a measure of an element’s tendency to undergo chemical reactions. It is determined by the number of valence electrons in an atom. The more valence electrons an atom has, the more reactive it is. The first 36 elements in the periodic table exhibit a wide range of reactivity, from highly reactive alkali metals to relatively inert noble gases.
Periodic table quiz 1 36 was a breeze! I’m curious about other topics now, like the Book of Mormon. Cal Poly has some interesting perspectives on it. Back to periodic table quiz 1 36, I’m determined to ace the next one!
Factors Influencing Reactivity
Several factors influence the reactivity of elements, including:
- Ionization energy:The energy required to remove an electron from an atom. The higher the ionization energy, the less reactive the element.
- Electronegativity:The ability of an atom to attract electrons in a chemical bond. The higher the electronegativity, the more reactive the element.
Examples of Chemical Reactions
Elements can undergo various chemical reactions, including:
- Combination reactions:Two or more elements combine to form a new compound. For example, hydrogen and oxygen react to form water: 2H 2+ O 2→ 2H 2O.
- Decomposition reactions:A compound breaks down into simpler substances. For example, water can decompose into hydrogen and oxygen: 2H 2O → 2H 2+ O 2.
- Single-replacement reactions:An element replaces another element in a compound. For example, iron reacts with copper sulfate to form iron sulfate and copper: Fe + CuSO 4→ FeSO 4+ Cu.
- Double-replacement reactions:Two compounds exchange ions to form two new compounds. For example, sodium chloride and silver nitrate react to form sodium nitrate and silver chloride: NaCl + AgNO 3→ NaNO 3+ AgCl.
Applications and Uses
The first 36 elements are essential for modern society, finding applications in diverse fields. These elements are incorporated into everyday products, industrial processes, and technological advancements.
Their unique properties and characteristics make them indispensable in various sectors, contributing to our daily lives, scientific breakthroughs, and technological innovations.
Industry
- Iron (Fe): Used in construction, transportation, and machinery due to its strength and durability.
- Aluminum (Al): Lightweight and corrosion-resistant, used in aircraft, vehicles, and construction.
- Copper (Cu): Excellent electrical and thermal conductivity, employed in wiring, electronics, and heat exchangers.
- Titanium (Ti): Strong, lightweight, and corrosion-resistant, used in aerospace, medical implants, and chemical processing.
- Zinc (Zn): Protects steel from corrosion, used in galvanizing and batteries.
Medicine
- Sodium (Na): Regulates fluid balance and nerve function, essential for electrolyte solutions.
- Potassium (K): Plays a crucial role in heart function and muscle contraction.
- Calcium (Ca): Essential for bone health, muscle function, and nerve transmission.
- Magnesium (Mg): Involved in over 300 enzymatic reactions, important for muscle function and energy production.
- Iodine (I): Necessary for thyroid hormone production, which regulates metabolism and growth.
Technology, Periodic table quiz 1 36
- Silicon (Si): The foundation of computer chips and semiconductors, enabling electronic devices.
- Germanium (Ge): Used in transistors and solar cells.
- Arsenic (As): Used in semiconductors and lasers.
- Gallium (Ga): Found in light-emitting diodes (LEDs) and solar cells.
- Selenium (Se): Used in photocopiers and solar cells.
FAQ Overview: Periodic Table Quiz 1 36
What is the periodic table?
The periodic table is a tabular arrangement of chemical elements, organized by their atomic number, electron configuration, and recurring chemical properties.
Why is the periodic table important?
The periodic table provides a systematic way to study the properties of elements and predict their behavior in chemical reactions.
What are the different groups and periods in the periodic table?
The periodic table is divided into 18 vertical columns called groups and 7 horizontal rows called periods.