Step into the realm of fluid mechanics, where density takes the spotlight! Density of water in slugs/ft3, a crucial concept, governs the behavior of fluids in captivating ways. Let’s dive into the depths of this fascinating topic, exploring its significance, units, conversions, and practical applications.
Unraveling the enigma of slugs and cubic feet, we’ll delve into the world of mass, force, and volume measurement. Prepare to be amazed as we unravel the formula for converting density from slugs/ft3 to other units, empowering you to navigate the fluid world with ease.
Density and its Significance
Density is a crucial concept in fluid mechanics. It refers to the mass per unit volume of a fluid and plays a significant role in determining the behavior of fluids.
The density of a fluid affects its buoyancy, pressure distribution, and flow patterns. For instance, denser fluids exert greater pressure at the bottom of a container than less dense fluids. Additionally, objects submerged in denser fluids experience greater buoyant forces, making them easier to float.
Applications of Density, Density of water in slugs/ft3
- Fluid Dynamics:Density is essential for understanding fluid flow and pressure distribution in pipelines, pumps, and turbines.
- Buoyancy and Flotation:Density determines the ability of objects to float or sink in a fluid, with denser objects sinking and less dense objects floating.
- Sedimentation and Filtration:Density differences between particles and fluids are utilized in sedimentation and filtration processes to separate solids from liquids.
- Meteorology and Oceanography:Density variations in the atmosphere and oceans drive weather patterns, ocean currents, and marine ecosystems.
Understanding Slugs and Cubic Feet
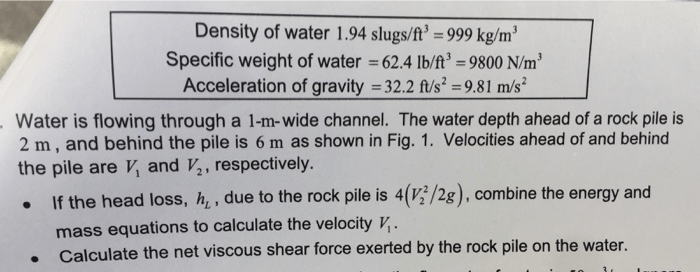
To delve deeper into the density of water expressed in slugs per cubic foot, it is essential to grasp the concepts of slugs and cubic feet as units of measurement.
The Unit “Slug”
The slug, denoted by the symbol “slug,” is a unit of mass in the English system of units. It is defined as the mass of an object that experiences a force of one pound-force when subjected to an acceleration of one foot per second squared.
In other words, a slug is a unit of mass equivalent to the mass of an object that weighs 32.174 pounds on Earth.
The Unit “Cubic Foot”
The cubic foot, abbreviated as “ft³,” is a unit of volume in the English system of units. It is defined as the volume of a cube with sides measuring one foot in length. The cubic foot is commonly used to measure the volume of three-dimensional objects, such as rooms, containers, and parcels.
Density Conversion
To facilitate comparisons and conversions across different measurement systems, it is crucial to understand the conversion process for density, especially when dealing with units like slugs/ft3. This conversion allows us to seamlessly switch between various density units, ensuring accurate and consistent data interpretation.
Conversion Formula
The fundamental formula for converting density from slugs/ft3 to other units is:
ρnew= ρ slugs/ft3× (Conversion Factor)
where:
- ρ newis the density in the desired unit (e.g., kg/m3, g/cm3)
- ρ slugs/ft3is the density in slugs/ft3
- Conversion Factor is a constant that depends on the desired unit
For example, to convert density from slugs/ft3 to kg/m3, the conversion factor is 515.379.
Conversion Steps
- Multiply the density in slugs/ft3 by the appropriate conversion factor.
- The result will be the density in the desired unit.
Applications of Density in Slugs/ft3: Density Of Water In Slugs/ft3
Density, expressed in slugs/ft3, finds widespread applications in various fields, particularly in engineering, physics, and hydrology. Its practical significance lies in its ability to quantify the mass distribution of substances, enabling engineers, scientists, and researchers to analyze and solve real-world problems.
Engineering
In engineering, density plays a crucial role in structural design and analysis. Engineers rely on density data to determine the weight and stability of structures, ensuring their safety and durability. For instance, in the design of bridges, buildings, and aircraft, engineers consider the density of materials used to calculate the overall mass and load-bearing capacity of the structure.
Physics
In physics, density is essential for understanding fluid dynamics, buoyancy, and other physical phenomena. For example, in the study of fluid flow, density determines the pressure exerted by fluids and their ability to resist motion. Similarly, in buoyancy calculations, the density of an object and the surrounding fluid influence its ability to float or sink.
The density of water in slugs/ft3 is a topic that has puzzled scientists for centuries. It is a complex issue that involves the interplay of several factors, including temperature, pressure, and salinity. Even hobbits, who are known for their love of the outdoors, are affected by the density of water.
In fact, it is one of the reasons why they don’t wear shoes. Why don’t hobbits wear shoes ? Because the density of water in slugs/ft3 is too high for their feet to handle.
Hydrology
In hydrology, density measurements are vital for studying water resources and understanding water flow patterns. Hydrologists use density data to determine the mass of water in rivers, lakes, and aquifers, aiding in water management and conservation efforts. Additionally, density variations in water bodies can indicate the presence of pollutants or changes in water quality.
Factors Affecting Density

The density of water, like any substance, is not a fixed property and can be influenced by various factors. Understanding these factors is crucial for accurate measurements and applications involving water density.
Three primary factors that significantly affect water density are temperature, pressure, and the presence of impurities.
Temperature
Temperature plays a vital role in determining water density. As water temperature increases, its density decreases. This is because the increased thermal energy causes water molecules to move faster and occupy more space, resulting in a decrease in mass per unit volume.
Pressure
Pressure also affects water density, albeit to a lesser extent compared to temperature. As pressure increases, water molecules are forced closer together, leading to an increase in density. However, this effect is typically observed at very high pressures, such as those encountered in deep-sea environments.
Impurities
The presence of impurities, such as dissolved salts or minerals, can alter the density of water. Impurities increase the mass of water without significantly affecting its volume, resulting in an increase in density. The extent of the density change depends on the concentration and type of impurities present.
Density Table for Water
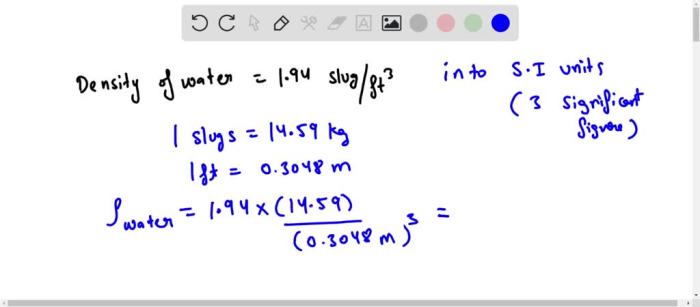
The density of water is a crucial property that varies with temperature and pressure. To provide a comprehensive understanding of this variation, we present a table summarizing the density values of water under different conditions.
Understanding the Table
The table lists the density of water in slugs per cubic foot (slugs/ft3) at various temperatures in degrees Fahrenheit (°F) and pressures in pounds per square inch (psi). This information allows engineers, scientists, and professionals to determine the density of water accurately for their specific applications.
Visual Representation of Density
The density of water varies depending on its temperature and pressure. This relationship can be visualized using a graph, which provides a clear and concise way to understand how density changes under different conditions.
Graphical Representation
The graph below shows the density of water over a range of temperatures and pressures. The x-axis represents the temperature in degrees Celsius, while the y-axis represents the density in slugs/ft3.
The graph shows that the density of water decreases as the temperature increases. This is because water molecules become more energetic at higher temperatures, causing them to move faster and take up more space. As a result, the same mass of water occupies a larger volume, resulting in a lower density.
The graph also shows that the density of water increases as the pressure increases. This is because pressure forces water molecules closer together, reducing the volume they occupy. As a result, the same mass of water occupies a smaller volume, resulting in a higher density.
General Inquiries
What is the significance of density in fluid mechanics?
Density plays a pivotal role in determining fluid behavior, influencing factors such as buoyancy, pressure distribution, and flow patterns.
How are slugs and cubic feet related to density?
Slugs represent mass, while cubic feet represent volume. Density is the ratio of mass to volume, expressed in slugs/ft3.
What are the practical applications of density in slugs/ft3?
Density finds applications in engineering design, fluid flow analysis, hydrology, and many other fields where understanding fluid behavior is crucial.