Welcome to the comprehensive guide to Unit 2 Review Chemistry Answers, where we delve into the fundamental principles, concepts, and applications of chemistry covered in Unit 2. Our aim is to provide a clear and concise exploration of key chemical concepts, empowering you with a deep understanding of this fascinating subject.
This guide covers a wide range of topics, including chemical bonding, chemical reactions, solutions, acids, bases, pH, gases, equilibrium, and thermodynamics. We will explore the different types of chemical bonds, their characteristics, and their impact on molecular properties. We will also investigate the various types of chemical reactions, their mechanisms, and the role of catalysts.
Furthermore, we will examine the properties of solutions, factors affecting their concentration, and their applications in various fields.
Unit 2 Chemistry Review
Unit 2 of Chemistry introduces the fundamental principles and concepts that govern chemical reactions and their applications. This unit delves into the study of chemical reactions, stoichiometry, and the behavior of gases.
Fundamental Principles of Chemistry
The fundamental principles of chemistry provide a framework for understanding the behavior of matter. These principles include the law of conservation of mass, which states that mass can neither be created nor destroyed during a chemical reaction. Additionally, the law of definite proportions states that a given compound always contains the same elements in the same proportions by mass.
Chemical Reactions
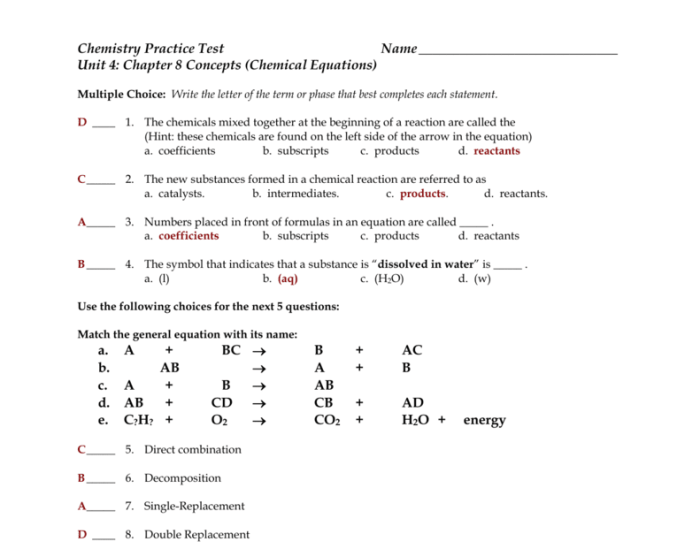
Chemical reactions involve the rearrangement of atoms and molecules to form new substances. These reactions can be classified into various types, including synthesis, decomposition, single displacement, double displacement, and combustion reactions. Each type of reaction follows specific patterns and has unique applications.
Stoichiometry
Stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It allows us to predict the amount of reactants and products involved in a given reaction. Stoichiometric calculations are essential for optimizing chemical processes and ensuring the efficient use of resources.
Chemical Bonding: Unit 2 Review Chemistry Answers
Chemical bonding is the process by which atoms, ions, or molecules are linked together to form chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction.
The strength of chemical bonds varies considerably; there are strong bonds such as covalent bonds, and weak bonds such as dipole-dipole interactions.
Chemical bonds are classified into different types based on the nature of the forces involved in their formation. The main types of chemical bonds are ionic bonds, covalent bonds, metallic bonds, and hydrogen bonds.
Ionic Bonds
Ionic bonds are formed between atoms of metals and nonmetals. In an ionic bond, one atom transfers one or more electrons to another atom. The atom that loses electrons becomes a positively charged ion, while the atom that gains electrons becomes a negatively charged ion.
The oppositely charged ions are attracted to each other by the electrostatic force, forming an ionic bond.
Ionic bonds are typically strong and result in the formation of crystalline solids. Ionic compounds are generally soluble in polar solvents such as water. Examples of ionic compounds include sodium chloride (NaCl), potassium chloride (KCl), and calcium fluoride (CaF 2).
Covalent Bonds
Covalent bonds are formed between atoms of nonmetals. In a covalent bond, the atoms share one or more pairs of electrons. The shared electrons are attracted to the nuclei of both atoms, forming a covalent bond.
Covalent bonds are typically weaker than ionic bonds and result in the formation of molecular substances. Covalent compounds are generally insoluble in polar solvents but soluble in nonpolar solvents. Examples of covalent compounds include hydrogen (H 2), methane (CH 4), and water (H 2O).
Metallic Bonds
Metallic bonds are formed between atoms of metals. In a metallic bond, the metal atoms share their valence electrons in a sea of electrons. The valence electrons are not attached to any particular atom but are free to move throughout the metal.
Metallic bonds are typically strong and result in the formation of metallic solids. Metallic solids are generally shiny, malleable, and ductile. Examples of metallic solids include copper (Cu), iron (Fe), and aluminum (Al).
Hydrogen Bonds
Hydrogen bonds are weak bonds that form between a hydrogen atom covalently bonded to an electronegative atom (such as oxygen, nitrogen, or fluorine) and another electronegative atom. The hydrogen atom in a hydrogen bond is partially positive, and the electronegative atom is partially negative.
The oppositely charged atoms are attracted to each other by the electrostatic force, forming a hydrogen bond.
Hydrogen bonds are weaker than ionic or covalent bonds but can play a significant role in determining the structure and properties of molecules. Hydrogen bonds are found in many biological molecules, such as DNA and proteins.
Chemical Reactions
Chemical reactions are processes that involve the rearrangement of atoms and molecules, leading to the formation of new substances. They play a crucial role in various aspects of life, including biological processes, industrial applications, and environmental transformations.
Chemical reactions can be classified based on their characteristics, such as the nature of the reactants and products, the energy changes involved, and the reaction mechanisms.
Types of Chemical Reactions
- Combination Reactions:Two or more substances combine to form a single product.
- Decomposition Reactions:A single substance breaks down into two or more products.
- Single-Displacement Reactions:One element replaces another element in a compound.
- Double-Displacement Reactions:Ions in two compounds exchange places, forming two new compounds.
- Combustion Reactions:A substance reacts with oxygen, releasing heat and light.
Mechanisms of Chemical Reactions
The mechanism of a chemical reaction refers to the specific steps involved in the transformation of reactants to products. Different types of reactions have distinct mechanisms:
Precipitation Reactions
In precipitation reactions, ions in solution combine to form an insoluble solid precipitate. The mechanism involves the formation of a supersaturated solution, followed by nucleation and crystal growth.
Acid-Base Reactions
Acid-base reactions involve the transfer of protons (H+) between acids and bases. The mechanism can be explained using the Brønsted-Lowry theory or the Lewis theory.
Redox Reactions
Redox reactions involve the transfer of electrons between reactants. The mechanism can be described using half-reactions, which show the oxidation and reduction processes separately.
Role of Catalysts
Catalysts are substances that increase the rate of a chemical reaction without being consumed in the reaction. They provide an alternative pathway for the reaction, lowering the activation energy required for the reaction to occur.
Solutions
Solutions are homogeneous mixtures of two or more chemical substances. The substance present in the largest amount is called the solvent, while the other substances are called solutes. Solutions can be in any of the three states of matter (solid, liquid, or gas).
The properties of solutions depend on the nature of the solvent and the solute. Some important properties of solutions include:
- Concentration: The concentration of a solution is a measure of the amount of solute present in a given amount of solvent. Concentration can be expressed in various units, such as molarity, molality, and mass percent.
- Colligative properties: Colligative properties are properties of solutions that depend only on the concentration of the solute particles, not on their identity. Colligative properties include freezing point depression, boiling point elevation, vapor pressure lowering, and osmotic pressure.
- Conductivity: The conductivity of a solution is a measure of its ability to conduct electricity. Solutions of electrolytes, which are substances that dissociate into ions in solution, have high conductivity. In contrast, solutions of non-electrolytes have low conductivity.
Factors Affecting Solution Concentration
The concentration of a solution can be affected by several factors, including:
- Temperature: The solubility of most solutes increases with increasing temperature. This is because higher temperatures provide more energy to overcome the intermolecular forces between the solute and solvent molecules.
- Pressure: The solubility of gases in liquids increases with increasing pressure. This is because higher pressures force more gas molecules into solution.
- Nature of solute and solvent: The solubility of a solute in a solvent depends on the chemical nature of both substances. Generally, solutes that are similar in polarity to the solvent will have higher solubility.
Applications of Solutions, Unit 2 review chemistry answers
Solutions have a wide range of applications in various fields, including:
- Chemistry: Solutions are used in many chemical reactions, such as titrations and extractions. They are also used to prepare standard solutions for calibration and analysis.
- Biology: Solutions are essential for life. The human body is made up of about 60% water, and many biological processes occur in aqueous solutions.
- Medicine: Solutions are used to deliver drugs to the body. They can be administered orally, intravenously, or topically.
- Industry: Solutions are used in a variety of industrial processes, such as electroplating, metalworking, and food processing.
Acids, Bases, and pH
Acids and bases are two important classes of chemical compounds that play a crucial role in many chemical reactions and biological processes. Acids are substances that release hydrogen ions (H+) in water, while bases are substances that release hydroxide ions (OH-) in water.
Properties of Acids and Bases
- Acidsare typically sour in taste, corrosive to metals, and react with bases to form salts and water.
- Basesare typically bitter in taste, slippery to the touch, and react with acids to form salts and water.
Strong and Weak Acids and Bases
Acids and bases can be classified as strong or weak depending on the extent to which they ionize in water. Strong acidsionize completely in water, releasing all of their hydrogen ions, while weak acidsionize only partially. Strong basesionize completely in water, releasing all of their hydroxide ions, while weak basesionize only partially.
Applications of Acids and Bases
Acids and bases have a wide range of applications in everyday life, including:
- Acidsare used in the production of fertilizers, dyes, plastics, and batteries.
- Basesare used in the production of soaps, detergents, and paper.
Gases
Gases are one of the four fundamental states of matter, characterized by their low density and ability to expand and fill their container. They play a crucial role in various scientific disciplines and industrial applications.
The properties of gases, such as pressure, volume, and temperature, are closely related to their kinetic molecular theory, which describes gases as collections of tiny particles in constant random motion.
Pressure
Gas pressure refers to the force exerted by gas particles on the walls of their container. It is directly proportional to the number of gas particles, their average kinetic energy, and inversely proportional to the volume of the container.
Volume
The volume of a gas is the space it occupies. According to Boyle’s law, the volume of a gas is inversely proportional to its pressure when temperature remains constant.
Temperature
Gas temperature is a measure of the average kinetic energy of its particles. According to Charles’s law, the volume of a gas is directly proportional to its absolute temperature when pressure remains constant.
Applications of Gases
Gases have numerous applications in various industries, including:
- Fuel: Natural gas, propane, and butane are commonly used as fuels for heating, cooking, and transportation.
- Industrial processes: Gases like hydrogen, oxygen, and nitrogen are used in chemical synthesis, metalworking, and food processing.
- Medical field: Oxygen, nitrous oxide, and helium are used in anesthesia, medical imaging, and respiratory therapy.
- Refrigeration: Gases like ammonia, chlorofluorocarbons (CFCs), and hydrofluorocarbons (HFCs) are used as refrigerants in cooling systems.
Equilibrium and Thermodynamics
Chemical equilibrium is a dynamic state in which the concentrations of reactants and products in a chemical reaction remain constant over time. It is a reversible process where the forward and reverse reactions occur at the same rate.Equilibrium is established when the Gibbs free energy of the system is minimized.
Factors that affect the equilibrium position include temperature, pressure, concentration, and the presence of a catalyst.
Applications of Equilibrium in Chemical Processes
Equilibrium plays a crucial role in various chemical processes, including:
-
-*Haber process
The production of ammonia from nitrogen and hydrogen.
-*Contact process
The production of sulfuric acid from sulfur dioxide.
-*Le Chatelier’s principle
Predicting the direction of a reaction when the equilibrium is disturbed.
-*Acid-base reactions
Determining the pH of a solution.
-*Solubility
Predicting the solubility of a substance in a solvent.
Quick FAQs
What are the fundamental principles of chemistry covered in Unit 2?
Unit 2 chemistry covers the basic principles of matter, its composition, structure, properties, and change. It explores the concepts of atomic structure, chemical bonding, chemical reactions, and energy changes involved in chemical processes.
How are chemical reactions classified?
Chemical reactions are classified based on their characteristics, such as the type of reactants and products involved, the energy changes that occur, and the mechanisms by which they proceed. Some common types of reactions include precipitation reactions, acid-base reactions, redox reactions, and combustion reactions.
What is the role of equilibrium in chemical processes?
Chemical equilibrium is a dynamic state in which the forward and reverse reactions of a chemical process occur at equal rates, resulting in no net change in the concentrations of the reactants and products. Equilibrium plays a crucial role in many chemical processes, including industrial processes, biological systems, and environmental chemistry.