Acids and bases virtual lab answers – Delve into the fascinating realm of acids and bases with our comprehensive virtual lab answers. Discover their fundamental concepts, explore their diverse applications, and unravel the secrets of their interactions. Embark on a journey of scientific exploration that will illuminate the intricate world of chemical reactions.
Our meticulously crafted virtual lab provides an immersive and interactive learning experience. Engage in hands-on experiments, analyze data, and draw insightful conclusions. Gain a deep understanding of acid-base chemistry and its profound impact on our daily lives.
1. Introduction to Acids and Bases
Acids and bases are two important concepts in chemistry that play a crucial role in various chemical reactions and everyday life.
Acids are substances that release hydrogen ions (H+) when dissolved in water, while bases are substances that release hydroxide ions (OH-) when dissolved in water.
1.1 Properties and Characteristics of Acids
- Sour taste
- Corrosive to metals
- React with bases to form salts and water
- Turn blue litmus paper red
1.2 Properties and Characteristics of Bases
- Bitter taste
- Slippery to touch
- React with acids to form salts and water
- Turn red litmus paper blue
1.3 Importance of Acids and Bases in Everyday Life
Acids and bases are essential for many everyday applications, such as:
- Batteries
- Fertilizers
- Food preservation
- Medical treatments
2. Acids and Bases Virtual Lab
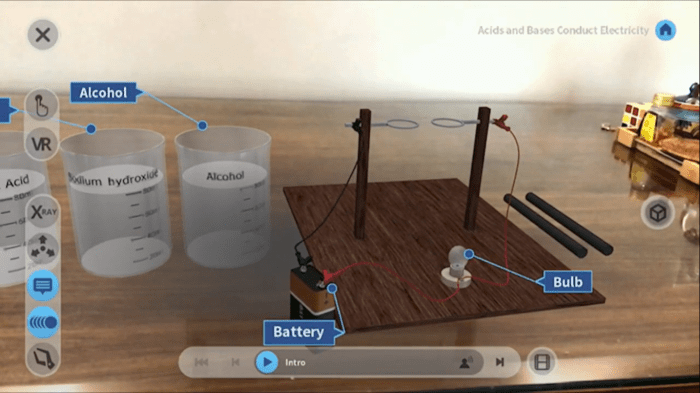
2.1 Overview of the Virtual Lab
The Acids and Bases Virtual Lab is an interactive online simulation that allows students to explore the properties and reactions of acids and bases.
The lab provides a safe and engaging environment to conduct experiments and observe the results in real-time.
2.2 Purpose and Objectives of the Lab
- To understand the concept of acids and bases
- To identify the properties and characteristics of acids and bases
- To observe the reactions between acids and bases
- To apply the knowledge of acids and bases to everyday life
2.3 How to Access and Use the Virtual Lab
The Acids and Bases Virtual Lab can be accessed through a web browser or a mobile app.
Once accessed, students can follow the instructions provided to conduct the experiments and record their observations.
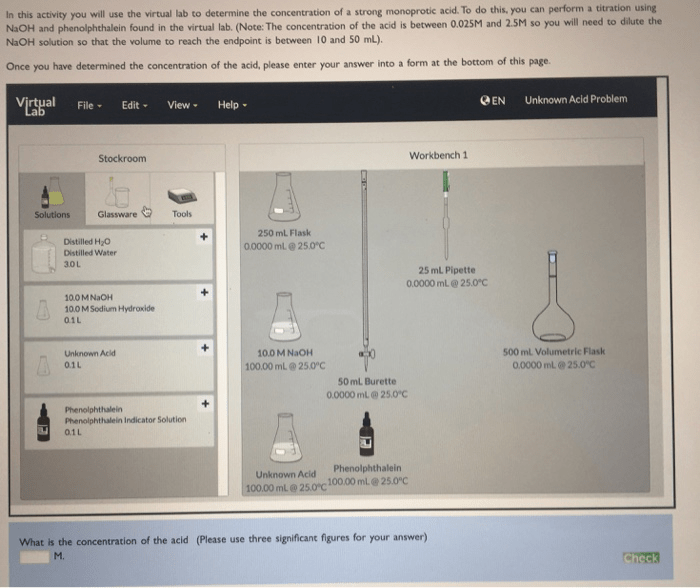
3. Lab Procedures and Experiments
3.1 Step-by-Step Guide for the Lab Procedures
- Log in to the virtual lab platform.
- Select the “Acids and Bases” experiment.
- Follow the instructions to prepare the virtual solutions.
- Conduct the experiments and record the observations.
- Analyze the data and draw conclusions.
3.2 Detailed Instructions for Each Experiment
The virtual lab includes a variety of experiments, each with its own set of instructions.
Students should carefully follow the instructions for each experiment to ensure accurate results.
3.3 Safety Precautions and Guidelines, Acids and bases virtual lab answers
- Wear appropriate safety gear, such as gloves and goggles.
- Do not ingest or inhale any chemicals.
- Dispose of chemicals properly according to the instructions provided.
- Follow the instructions of the lab instructor or supervisor.
4. Data Analysis and Interpretation: Acids And Bases Virtual Lab Answers
4.1 Table to Organize and Present Experimental Data
| Experiment | Observations | Conclusions |
|---|---|---|
| Reaction of HCl with NaOH | Formation of NaCl and water | HCl is an acid and NaOH is a base |
| Reaction of CH3COOH with NaOH | Formation of CH3COONa and water | CH3COOH is a weak acid and NaOH is a strong base |
4.2 How to Analyze the Data
Students should analyze the experimental data to identify patterns and trends.
They should also compare their results to the expected outcomes to draw conclusions about the properties and reactions of acids and bases.
4.3 Significance of the Results
The results of the experiments help students understand the fundamental principles of acids and bases.
They also provide evidence to support the theoretical concepts discussed in the introduction.
5. Applications of Acids and Bases
5.1 Real-World Applications of Acids and Bases
Acids and bases have a wide range of applications in various industries, including:
- Industry: Production of fertilizers, plastics, and batteries
- Medicine: Treatment of stomach ulcers, heartburn, and infections
- Everyday life: Cleaning products, food preservation, and water treatment
5.2 Examples of How Acids and Bases Impact Our Daily Lives
- The acid in batteries provides the electrical energy for our devices.
- The base in soap helps to remove dirt and oil from our skin.
- The acid in lemon juice adds flavor to our food.
Key Questions Answered
What are the key characteristics of acids?
Acids are substances that release hydrogen ions (H+) when dissolved in water. They typically have a sour taste, turn blue litmus paper red, and react with metals to produce hydrogen gas.
How can I identify a base?
Bases are substances that release hydroxide ions (OH-) when dissolved in water. They have a bitter taste, turn red litmus paper blue, and feel slippery to the touch.
What is the purpose of a neutralization reaction?
A neutralization reaction is a chemical reaction between an acid and a base that results in the formation of salt and water. This reaction is used to neutralize the effects of acids or bases in various applications.