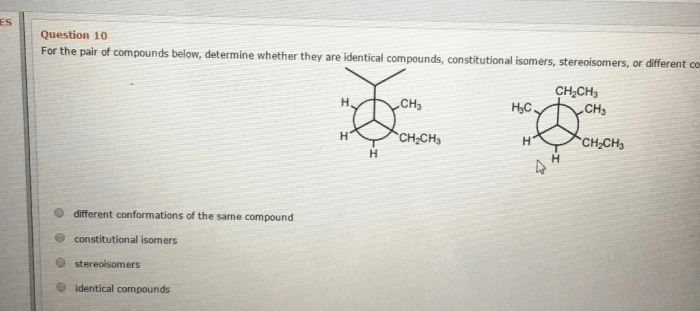
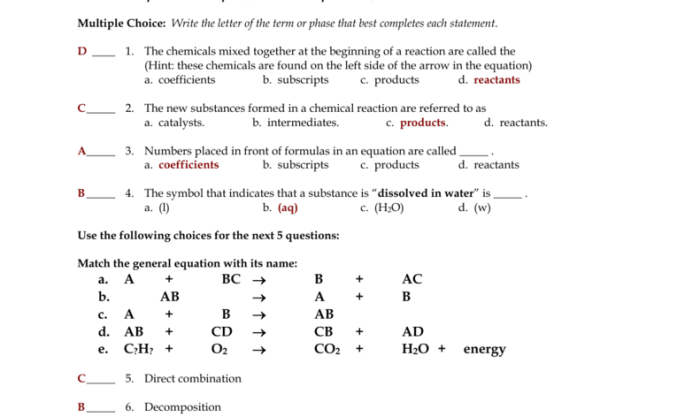
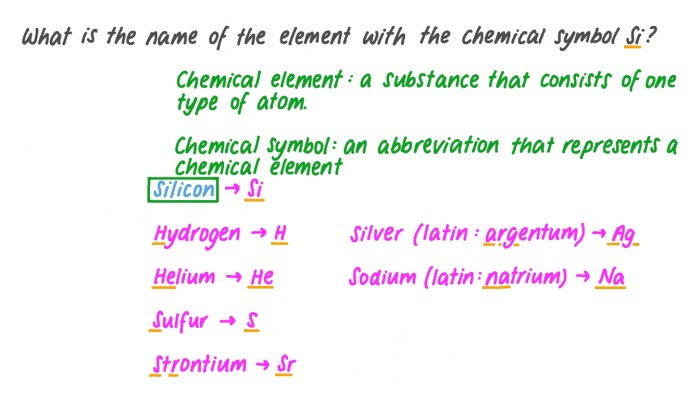
Predicting products chemical reactions worksheet answers is a crucial aspect of chemistry that enables scientists to understand and manipulate chemical processes. This guide provides a comprehensive overview of the methods, applications, and challenges associated with predicting the products of chemical reactions, empowering students and professionals alike to delve into the intricacies of this fascinating field.
Predicting the products of chemical reactions is essential for designing new materials, developing efficient processes, and understanding the behavior of chemical systems. However, it can be a complex task, influenced by various factors such as the nature of the reactants, reaction conditions, and the presence of catalysts.
This guide will explore the different methods used to predict reaction products, their advantages and disadvantages, and the factors that affect their accuracy.
Introduction: Predicting Products Chemical Reactions Worksheet Answers

Chemical reactions are processes that involve the rearrangement of atoms and molecules, resulting in the formation of new substances. Predicting the products of chemical reactions is crucial in various scientific and engineering fields, as it enables scientists and engineers to design new materials and processes, understand chemical behavior, and assess potential risks and benefits.
However, predicting products of chemical reactions can be challenging due to the complexity of chemical systems and the numerous factors that can influence the outcome of a reaction. Therefore, it is essential to understand the different methods available for predicting products and their respective advantages and disadvantages.
Methods for Predicting Products of Chemical Reactions
There are several methods available for predicting products of chemical reactions. Each method has its own advantages and disadvantages, and the choice of method depends on the specific reaction being studied and the available information.
- Balanced chemical equations: A balanced chemical equation provides information about the stoichiometry of a reaction, indicating the relative amounts of reactants and products involved. By analyzing the coefficients in the balanced equation, one can determine the products of the reaction.
- Thermodynamics: Thermodynamics can be used to predict the spontaneity of a reaction and the equilibrium concentrations of the products. By calculating the Gibbs free energy change (ΔG) of a reaction, one can determine whether the reaction is thermodynamically favorable and predict the products that will be formed at equilibrium.
- Kinetics: Kinetics studies the rates of chemical reactions and can be used to predict the products of a reaction by considering the relative rates of competing reactions. The reaction with the fastest rate is more likely to occur and produce the corresponding products.
- Computational chemistry: Computational chemistry methods, such as density functional theory (DFT) and molecular dynamics simulations, can be used to simulate chemical reactions and predict the products. These methods provide detailed information about the reaction pathway, including the transition states and intermediate species.
The accuracy of each method depends on various factors, such as the complexity of the reaction, the availability of experimental data, and the computational resources available.
Examples of Predicting Products of Chemical Reactions
The following are examples of how different methods can be used to predict products of chemical reactions:
- Combustion of methane: The balanced chemical equation for the combustion of methane is CH 4+ 2O 2→ CO 2+ 2H 2O. This equation indicates that methane will react with oxygen to produce carbon dioxide and water as the products.
- Acid-base neutralization: The reaction between a strong acid and a strong base is a neutralization reaction that produces salt and water. For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) can be predicted using the balanced equation: HCl + NaOH → NaCl + H 2O.
- Precipitation reactions: Precipitation reactions occur when two soluble ionic compounds react to form an insoluble solid precipitate. For example, the reaction between silver nitrate (AgNO 3) and sodium chloride (NaCl) can be predicted using the balanced equation: AgNO 3+ NaCl → AgCl (s) + NaNO 3.
These examples illustrate how different methods can be used to predict the products of chemical reactions. The choice of method depends on the specific reaction being studied and the available information.
Applications of Predicting Products of Chemical Reactions, Predicting products chemical reactions worksheet answers
Predicting products of chemical reactions has numerous applications in various fields, including:
- Chemistry: Predicting products of chemical reactions is essential for understanding chemical behavior, designing new materials and processes, and developing new drugs and therapies.
- Engineering: Chemical engineers use product prediction to design chemical plants and processes, optimize reaction conditions, and assess the safety and environmental impact of chemical reactions.
- Environmental science: Predicting products of chemical reactions is crucial for understanding environmental processes, such as the fate and transport of pollutants, the formation of smog, and the impact of climate change.
- Materials science: Materials scientists use product prediction to design new materials with desired properties, such as strength, durability, and conductivity.
- Pharmacology: Predicting products of chemical reactions is essential for developing new drugs and understanding their metabolism and interactions with the body.
Overall, predicting products of chemical reactions is a powerful tool that enables scientists and engineers to understand chemical behavior, design new materials and processes, and address challenges in various fields.
Quick FAQs
What are the different methods for predicting the products of chemical reactions?
Common methods include balancing chemical equations, using the periodic table, considering reaction types, and employing computational tools.
What factors affect the accuracy of product prediction methods?
Factors include the complexity of the reaction, the availability of thermodynamic data, and the presence of side reactions.
What are the applications of predicting the products of chemical reactions?
Applications include designing new materials, developing efficient processes, and understanding the behavior of chemical systems.